Chemistry of ethyne
Introcudtion
Molecular formula = C2H2
Empirical formula = CH
Molecular mass = 26
Empirical mass = 13
Common name = Acetylene
Homologous series = Alkynes
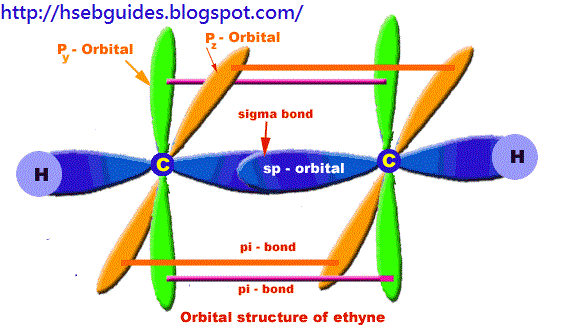
Orbital structure of ethyne
Compostion of ethyne molecule:
Ethyne molecule consists of two C-atoms and two H-atoms (C2H2).
Nature of hybridization:
In ethyne molecule, each carbon atom is Sp-hybridized. Due to Sp-hybridization, each carbon atom generates two Sp-hybrid orbitals. In this way there exists four Sp-orbital in ethyne. These Sp-orbital are arranged in linear geometry and 180o apart. Remaining py and pz unhybrid orbitals of each carbon atom lie perpendicular to the plane of Sp-orbitals.
Sigma bond formation:
One Sp-hybrid orbital of each carbon atom overlaps to produce one sigma bond between two C-atoms. The remaining one Sp-orbital of each C-atom overlaps with one H-atom to produce sigma bond.
Pi-bond formation:
Py and Pz orbital of two carbon atoms are un-hybrid and make parallel overlapping to produce pi-bond.
Bond length:
The C–H bond is 1.09A and C-C is 1.2A.o.
Bond angle:
HCC bond angle is 180o.
Methods of preparation
By the hydroclysis of calcium carbide:
CaC2 + 2H2O == C2H2 + Ca(OH)2
By tetra chloroethane:
Cl2CH-CHCl2 + 2Zn == C2H2 + 2ZnCl2
By 1,2-dibromoethane:
Br-CH2-CH2-Br + KOH == C2H2 + 2KBr + 2H2O
Chemical properties
Combustion reaction:
2C2H2 + 5O2 == 4CO2 + 2H2O + heat
Addition reaction:
Addition of Hydrogen:
C2H2 == CH2=CH2 == C2H6
Addition of halogen:
C2H2 +Cl2 == Cl-CH=CH-Cl == Cl2CH-CHCl2
Addition of hydrogen halide:
C2H2 + HBr == CH2=CH-Br + HBr == CH3-CHBr2
Addition of HCN:
C2H2 + HCN == CN-CH=CH-CN
Addition of water:
C2H2 + HOH == CH2=CH-OH == [rearrangement] == CH3CHO (Ethanal)
Oxidation of ethyne:
In cold solution
C2H2 + HOH + 3[O] == 2HCOOH (Formic acid)
In hot solution,
C2H2 +4[O] == (COOH)2 (Oxalic acid)
Substitution reactions:
Consult your text book
Physical properties
- Ethyne is a colourless gas with a characteristic smell.
- It has a melting point -81oC.
- It has a boiling point -84oC.
- It is significantly soluble in water. However, readily soluble in organic solvents.
Uses of ethyne
- It is used in welding to produce oxy-acetylene flame of temperature about 3000oC.
- It is used in the synthesis of useful compounds such as ethanol, ethanoic acid, PVC, acetaldehyde.



Top comments (0)