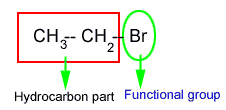
Functional group
An atom or group of atoms or radical which is responsible for the entire chemical properties of an organic compound is called a functional group.
Or
An atom or group of atoms in a molecule that gives the molecule
its characteristic chemical properties is called a functional group.
Or
Active part of an organic compound which takes part in chemical reaction is always a functional group.
Generally an organic compound is composed of two parts:
- Hydrocarbon part
- Active part
Hydrocarbon part of an organic compound is chemically inert and the other part which is active is called functional group.
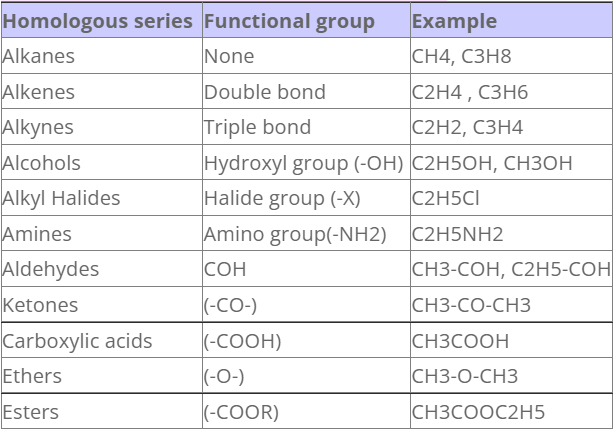
Organic compounds and their functional groups
Types of organic compounds
Organic compounds are divided on to two major categories:
- Open chain compounds or acyclic compounds
- Closed chain compounds or cyclic compounds
Open chain compounds
Organic compounds which have open chain skeleton are known as aliphatic compounds.
For example:
Closed chain compounds
Organic compounds containing closed ring are known as cyclic compounds.
For example:
Types of cyclic compounds
Homocyclic or carbocyclic compounds:
When ring of compound consists of carbon atoms only, the compound is said to be “homocyclic or carbocyclic compound”.
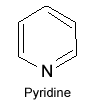
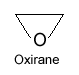
Heterocyclic compounds
When ring of compound consist of carbon as well as any other element, the compound is said to be “heterocyclic compounds”.
Or
Cyclic compounds which contain at least one atom of another element in addition to carbon in the ring are referred to as “heterocyclic compounds”.
Types of homocyclic compounds
Homocyclic compounds are divided into two types:
- Alicyclic compounds
- Aromatic compounds
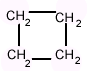
Alicyclic compounds
Homocyclic compounds which resemble aliphatic compounds and do not contain benzene ring are termed as “Alicyclic compounds”.
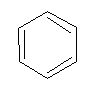
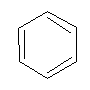
Aromatic compounds
Homocyclic compounds containing benzene ring are known as “Aromatic compounds”.
Cracking
When high molecular mass long chain alkanes are subjected to heat above their boiling point, they are broken down to produce smaller alkanes, alkenes and hydrogen. This thermal decomposition is called “cracking”.
Cracking involves splitting of larger alkanes of less volatile into smaller molecules of high volatility on heating at high temperature and pressure in the presence of catalyst.
Types of cracking
- Thermal cracking
- Catalytic cracking
- Catalytic cracking
In catalytic cracking, a catalyst such as silica or aluminum or zeolite is used.
Thermal cracking
2CH3-CH2-CH3 –> CH3-CH=CH2 + CH2=CH2+CH4 +H2
Industrial applications of cracking
- Gasoline Production
- Petrochemical Production
- Fuel gas Production
- Synthetic petrol Production









Top comments (0)