Gas molecules are in a state of constant motion. So, they intermix with each other to form a homogeneous mixture. Diffusion is a process by virtue of which two or more gases intermix with each other, independent of gravitation to form a homogeneous mixture.
Example: If hydrogen sulphide (H2S) is released at one corner of room, its smell can be detected at another corner after some time.
Statement of Graham’s Law of Diffusion:
Under identical conditions of temperature and pressure, the rate of diffusion of gases is inversely proportional to the square root of their densities.
If r1 and r2 are the rates of diffusion of any two gases whose densities are d1 and d2 respectively, then according to this law:
Hence, Graham’s Law of diffusion can also be stated as the rate of diffusion of gases is inversely proportional to the square root of their molecular masses.
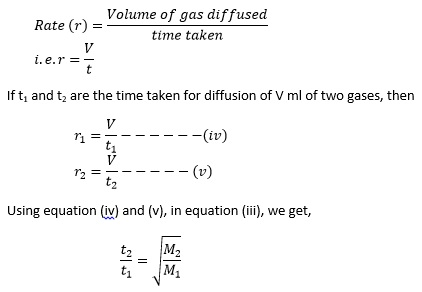
Rate of diffusion is defined as the volume of gas diffused per unit time.
The time taken for diffusion is directly proportional to the molecular weight.
Experimental verification of Graham’s Law:
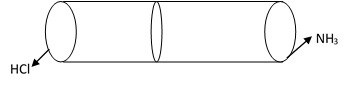
Graham’s Law of Diffusion can be verified by smoke ring experiment.
In this experiment a gas tube which is open at both ends is taken. At one end a cotton plug dipped in HCl is fitted and at other end a cotton plug dipped in NH3 is fitted. The vapor of NH3 and HCl diffused in opposite direction, and they form a ring of white dense fune of NH4Cl. It is found that the distance of the ring is closer to the end containing HCl than that of the end containing NH3. This verifies Graham’s Law of Diffusion.
Application of Graham’s Law of Diffusion:
- To determine the molecular mass and vapor density of a gas.
- To separate different gases from their mixture.
- To separate the isotopes of same element.

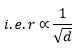
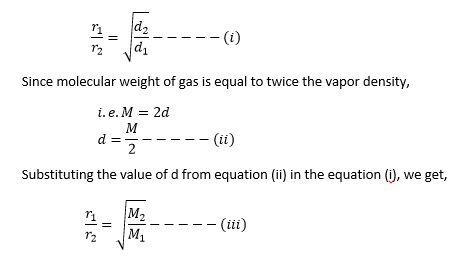

Top comments (0)