Theory:
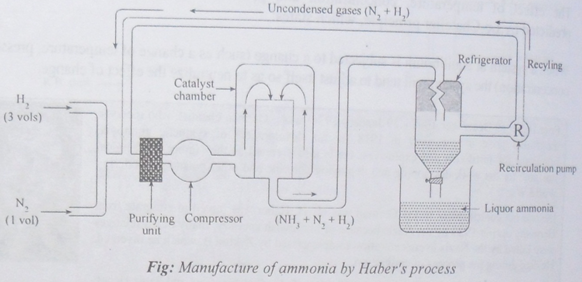
NH3 is manufactured by Haber’s process. In this process, pure nitrogen and hydrogen gases react in the ratio of 1:3 by volume in 400°C-500°C temperature, 200-900 atm pressure in presence of iron as catalyst & molybdenum as promoter.
Fe/Mo (400-500°C)
N2+3H2 ——————–> 2NH3+ heat
(200-900atm)
Conditions for maximum yield:
1. Low temperature:
Since the reaction isexothermic, it favors low working temperature for maximum yield of product i.e. around 500°C.
2. High pressure:
Four volume reactant gives two volume of product i.e. volume is reduced to half. Hence, there is maximum production of ammonia at 200-900 atm pressure (high).
3. Use of catalyst:
Use of catalyst makes the reaction faster in forward direction. Hence, the catalyst like iron (Fe) & promoter like molybdenum (Mo) is used for maximum yield of the product.
4. Purity of reactant:
The purity of the reactants Hydrogen (H2) & Nitrogen (N2) helps in the more yielding of the product.
5. Concentration of reactants:
More the concentration of reactant, more the production. So, high concentration of hydrogen and nitrogen is used.


Top comments (0)