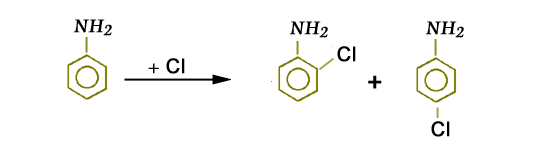
Some groups direct the second incoming group to the ortho and para positions simultaneously. These groups are called ortho-para directors. For example, when aniline is chlorinated, the product so obtained is ortho and para.
The (-NH2) group directs the incoming group -Cl to ortho and para positions on the ring. Therefore,
(-NH2) group is an ortho-para director. Some common ortho para directing groups are –Cl, -Br, -I, -OH, -NH2, -CH3, -C2H5.
Some ortho para directors
Halogens , -NH2, -R, -OH
Meta directing effect
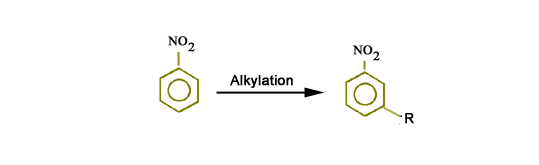
The group which directs the second incoming group to the meta position, is called a meta-director.
For example, alkylation of nitro benzene gives m-alkylnitro benzene as major product.
Therefore, –NO2 group is a meta director.
Some meta directors
-NO2, -CHO, -COOH, COOR, –SO3H, -CN, -COR etc.
Effects of substituents on reactivity
Substituents already present on a benzene ring also influences the rate of reaction.
For example, toluene, C6H5-CH3, is nitrated 25 times faster than benzene itself. On the other hand, the rate of nitration of chlorobenzene, C6H5-Cl is 30 times less than benzene. This means that the presence of CH3 on benzene ring activates it to aromatic electrophilic substitution, while the presence of a (-Cl) group deactivates it.
A substituent, which activates the aromatic ring to further substitution, is called an activating substituent or ring-activator.
A substituent, which deactivates the aromatic ring to further substitution, is called a deactivating substituent or ring-deactivator


Oldest comments (0)