Momomer
Single repeating unit of a large molecule or polymer is called a “monomer”.
Ploymer
A high molecular mass compound, which consist of repeating units, is called “polymer”.
Polymerization
A self-addition reaction in which a number of simple molecules (monomers) are joined to form a very large molecule is called polymerization.
Or
A chemical reaction in which monomers are converted into polymer is called polymerization.
For example:
n(C2H4) (-CH2-CH2-CH2-) or (-CH2-CH2-)n monomer polymer
(ethylene) (polyethene)
Types of polymerization
There are two types of polymerization:
- Addition polymerization
- Condensation polymerization
1. Addition polymerization
When a number of monomers undergo self-combination, resulting substance has a molecular mass many times larger than the monomer, the type of polymerization is known as “ Addition polymerization”.
The empirical formula of addition polymer and the monomer is same.
These reactions are catalyzed by peroxides or acids.
Generally these reactions required a pressure of 100 atmosphere at 200oC.
Examples: polyethylene, polypropylene, P.V. C(Poly Vinyl Chloride), teflone.
2. Condensation polymerization
Condensation polymers are formed by the combination of monomers with the elimination of simple molecules such as H2O or CH3OH.
There are two types of Condensation polymers:
- Polyester.
- Polyamide. Examples: Nylon, terylene, Bakelite.
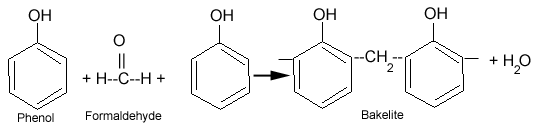
What is Bakelite?
Bakelite is a condensation polymer of phenol and formaldehyde. Bakelite is widely used for making molded products.
Vital force theory
In old days, it was believed that organic compounds could be produced only by living matte. It was also assumed that living matter possess a mysterious or vital force which converts it into organic compounds and only living matter can produce organic compounds.
Failure of vital force theory
Vital force theory has failed now because a number of organic compounds can be prepared by inorganic or non-living stuff. Fredrick wholer, a German chemist was the first person who, in 1828 prepared an organic substance urea by using inorganic substance Ammonium Cyanate by boiling with water.
Catenation
Carbon has ability to form bonds successively to other C-atoms to form chains of varying lengths, structures and shapes. This property of carbon is called “Catenation”. Catenation is responsible for the variety and a large number of organic compounds.
Due to catenation carbon forms long chains, branched chains, rings, ring and chain structured compounds.

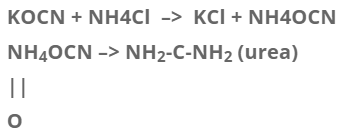

Top comments (0)