For ortho-para directors
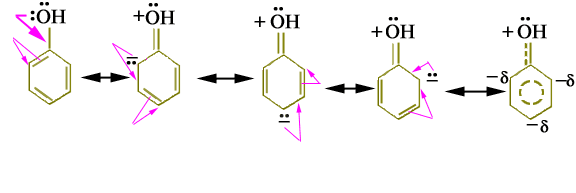
The resonance theory explains why certain substituents are ortho-para directing and some are meta-directing. Let us discuss various resonance forms of phenol in which -OH group is already attached to the benzene ring.
Directive effects
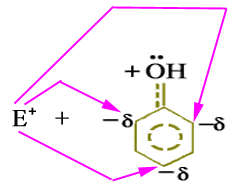
There are two non-bonding electrons pairs on the oxygen-atom of -OH group attached to the ring. One of these is distributed into the ring by the interaction with the p-system. In resonance forms the ortho and para positions have a greater electron density than the meta positions. Therefore, the resonance hybrid has negative charges in the ortho and para positions with the electron delocalization. The electrophile (E+) would naturally attack at these electron-rich centers, regardless of its nature.
Above discussion indicates that -OH group directs the incoming group to ortho and para positions and is an ortho and para director.


Top comments (0)