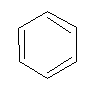
What is the exact structure of benzene?
Kekule's structures for benzene
To answer this question a chemist KEKULE, in 1865 suggested the following structure for benzene.
Kekule’s structure of benzene:
According to Kekule:
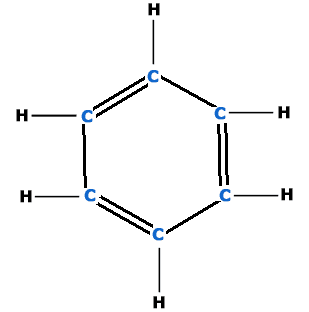
- Six carbon atoms in benzene are on the six corners of a regular hexagone.
- Each C-atoms is attached with one H-atom.
- There are 3 alternate double bonds between two C-atoms to complete fourth valency of carbon i.e.
Objections to kekule's proposed structure
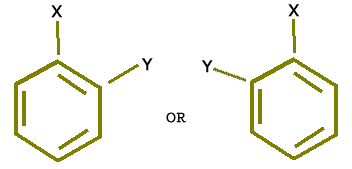
There was an objection to the above mentioned structure of benzene that it must give two ortho isomers (Di substitution) but in actual practice only one ortho isomer is obtained i.e.
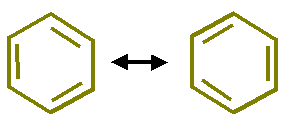
To answer this question Kekule extended his theory and argue that the position of double bond in benzene is not fixed but the double bond system is revolving over the hexagonal ring. He proposed following structures and said that all C-C positions have a partial double bond character.
In the extension of his theory, Kekule, explained that C-atoms in benzene were in a state of vibration and due to this vibration each pair of C-atoms has a single bond half of the time and a double bond the other half time.
Kekule’s structure failed to explain why benzene with three double bonds does not undergo addition reactions like alkenes or alkynes.
Q: Define aromaticity?
A: The aromatic compounds containing alternate double and single bonds in a cyclic structure and resemble benzene in chemical behaviour. They undergo substitution reactions rather addition reactions. The characteristic behaviour is called Aromaticity or aromatic character. Criteria of Aromaticity:
An aromatic compound is cyclic and planar.
Each atom in an aromatic cyclic ring has a p-orbital. These orbital are parallel to each other and continuous overlapping is possible around the ring.
The cyclic pi-molecular orbital formed by the overlapping of p-orbital must contain (4n+2) pi-electrons. This is called huckle rule. Where ‘n’ is an integer (0,1,2,3,…)


Top comments (0)