Statical and frictional electricity
The energy produced after rubbing two objects is called static electricity. It is the friction between the two objects that generate attraction between the objects leading to charge transfer. It is due to this contribution of friction that static energy is also called frictional electricity.
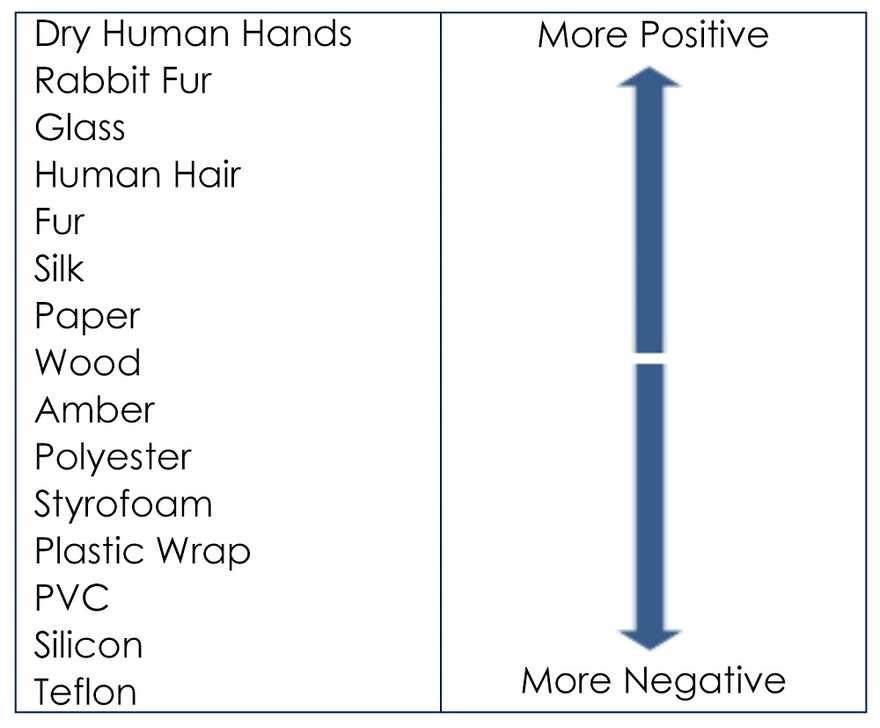
When two objects are rubbed together we often see the exchange of electrons that is why you might have felt a quick shock while touching a metal doorknob or any other such items often when moving around a carpeted floor. This phenomenon is well explained by the triboelectric effect. When two objects are rubbed against one another if one object loses the electrons other is to gain it and vice versa. The table below lists the items which lose electrons (positive) and ones which gain electrons (negative):
Electrostatic induction
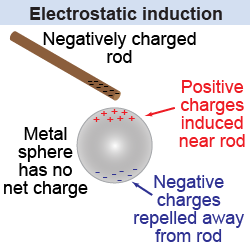
Electrostatic induction is a process of generating static electricity by bringing an electrically charged object near a material. While doing so, the electrical charges stored within the material is redistributed causing one side to have more of either +ve (positive) or -ve (negative) charge.
Conductors and insulators
Electric current can flow freely in a conductor while the electric current can’t flow freely in an insulator. Examples of the conductor can be metals like copper. Non-metallic solids are examples of an insulator. These non-metallic solids have extremely high resistance to the flow of charge through them and are thus said to be good insulators.
Free electrons and bound electrons
Imagine a universe with no stars and just planets roaming freely, this scenario can be an example of free electrons. Thus, any electron which is not attached to an atom, molecule or an ion and is free to move under the influence of an applied electric or magnetic field is called as free electrons.
While a bound electron is an electron that move around a specific atom or crystal due to electromagnetism.


Top comments (0)