Heat is a form of energy and is defined as:
The total kinetic energy of all the molecules of a body.
Symbol
It is denoted by “Q” or “q”
Units
- Joule
- B.T.U
- Calorie
Conversion
1 B.T.U = 251.996 Cal ( or 252 Cal)
1 calorie = 4.186 Joule ( or 4.2 joule)
Flow of heat
Natural flow of heat always takes place from a region of high temperature to a region of lower temperature.
Thermometric properties
Properties of a substance that vary uniformly with the variation in temperature are referred to as Thermometric properties.
Examples:
Volume , Density ,Viscosity , Pressure , Surface Tension , Area
Thermal equilibrium
When two bodies at different temperatures are brought into thermal contact , heat flows from hot
body to cold body till the temperature of both the bodies becomes equal . This state is referred to as thermal equilibrium.
Temperature
Average kinetic energy of the molecules of a body is called temperature.” Temperature describes the degree of hotness of a body.

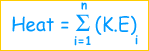

Top comments (0)