In classical mechanics position co-ordinates, component of momentum, components of angular momentum are measured in an arbiter precision but in quantum mechanics particle is considered as wave and components of position and momentum are measured with highly improved precision as compared to classical mechanics.
The amplitude of the wave remaining almost zero in wide range of wave but specific value in narrow region of wave indicates high precision in position of the particle measured as momentum is conjugate quantity with position, high precision in position reflects worst precision in momentum. Hence, Heisenberg states that ‘it is impossible to determine position and momentum simultaneously with 100% accuracy.
These quantities are related as:
This equation was further improved by Heisenberg which can be expressed as:
Uncertainty principle proposed by Heisenberg was further modified by Kennard and obtained the result as:
The most important thing is that uncertainty principle holds true for both microscopic and microscopic particle and it is independent with the method of measurement.


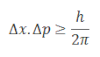
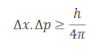

Oldest comments (0)