Isochoric process
A thermodynamic process in which the volume of the system remains constant during the supply of heat is called an isochoric process.
Explanation
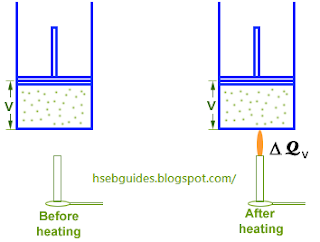
Consider a cylinder fitted with a frictionless piston. An ideal gas is enclosed in the cylinder. The piston is fixed at a particular position so that the volume of cylinder remains constant during the supply of heat.
Let DQ amount of heat is added to the system. Addition of heat causes the following changes in the system:
- Internal energy increases from U1 to U2.
- Volume of the system remains unchanged.
- Temperature increases from T1 K to T2 K.
- Pressure increases from P1 to P2.
- No work is performed.
According to the first law of thermodynamics:
DQ = DU+ DW
But DW = PDV
Thus,
DQ = DU+ PDV As
DV = 0
DQ = DU+ P (0)
DQ = DU
This expression indicates that the heat supplied under isochoric process is consumed in increasing the internal energy of the system but no work is performed.
Graphical representation
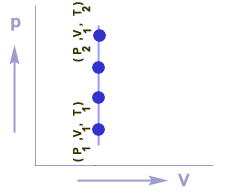
Graph between P & V for an isochoric process is a straight line which is parallel to P-axis.


Top comments (0)