Specific heat
“Amount of heat energy required to raise the temperature of unit mass of a substance by one Kelvin or 1° C is known as specific heat of the substance.”
Mathematical expression
If D Q is the amount of heat required to raise the temperature of “m” kg of the substance through ‘D T Kelvin , Then
D Q µ m …….(i)
D Q µ D T ……..(ii)
or D Q = m c D T
where ‘C’ is the constant called specific heat of substance.
Or
c = D Q / m D T
Unit of specific heat
Unit of specific heat is J/kg K in S.I. system
Experiments show that specific heat of a particular material varies with temperature.
Specific heat is not a precious concept for calculation because numbers of molecules per unit mass changes from material to material.
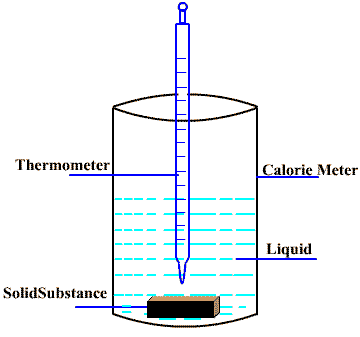
Determination of specific heat
Specific heat of a solid substance can be determined by the “Method of Mixture” using the concept of the “law of Heat Exchange” i.e.
heat lost by hot body = heat gained by cold body
The method of mixture based on the fact that when a hot substance is mixed with a cold substance, the hot body loses heat and the cold body absorbs heat until thermal equilibrium is attained. At equilibrium, final temperature of mixture is measured. The specific heat of the substance is calculated with the help of the law of heat exchange.
Let,
Mass of substance = ms kg
Mass of liquid = ml kg
Mass of calorie meter = mc kg
Initial temperature of the substance = Ts K
Initial temperature of the liquid = Tl K
Initial temperature of the calorie meter = Tc K
Specific heat of substance = Cs = ?
Specific heat of liquid = Cl
Specific heat of the material of the calorie meter = Cc
Final temperature of the mixture = T K
According to the law of heat exchange:
Which is the required value of specific heat of solid in J/ kg K.

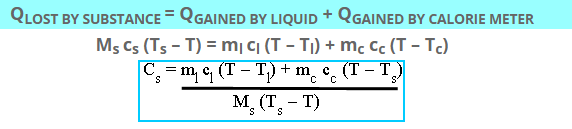

Top comments (0)