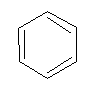
Introduction
- Molecular formula = C6H6
- Empirical formula = CH
- Molecular mass = 78
- Empirical formula mass = 13
- Percentage of carbon = 93.6%
- Percentage of hydrogen = 6.4%
It is an aromatic hydrocarbon.
Physical state = liquid at room temperature.
Melting point = 5.5oC
Boiling point = 80oC
It is highly inflammable.
Benzene burns with smoke due to high percentage of carbon.
It is insoluble in water.
It is soluble in organic solvents.
Benzene dissolves fats, sulphur, iodine and resins.
Nature:
- highly unsaturated (according to structure)
- saturated (according to reactions)
- Nature of reactions:
- Substitution reactions
- Addition reactions (under special conditions)
- Benzene was first isolated by Faraday.
- Hoffman was the first scientist who separated benzene from coal tar.


Top comments (0)