Saturated hydrocarbons
Hydrocarbons in which all the valencies of carbon atoms are fully utilized by single covalent bonds are known as saturated hydrocarbons.
Reactivity
They are saturated in respect to chemical combination with the other compounds and elements. Saturated hydrocarbons are chemically inert to some extent.
e.g. Alkanes (methane, ethane, propane etc.)
Characteristic reactions:
Characteristic reactions of saturated hydrocarbons are “substitution reaction”.
Addition reactions:
They do not undergo addition reactions in any circumstances.
Unsaturated hydrocarbons
Hydrocarbons in which all the valencies of carbon atoms are not fully utilized by single covalent bonds are known as “unsaturated hydrocarbons”.
They contain at least one double or triple bond in their structure.
e.g. Alkenes, Alkynes
CH2=CH2
Reactivity:
They are very reactive hydrocarbons. Their high reactivity is due to the presence of pi-bond in their structure.
Characteristic reactions:
Their characteristic reactions are addition reactions.
Substitution reactions:
Unsaturated hydrocarbons (alkynes) may undergo substitution reactions.
Alkanes
Alkanes are open chain saturated hydrocarbons (aliphatic hydrocarbons) in which all carbon atoms are bonded to each other by single covalent bond. Each carbon is tetrahedrally surrounded by H-atoms. Since all the valencies of carbon atoms are fully utilized by sigma bond with H-atoms therefore, they
are also known as “saturated hydrocarbons”.
General formula: CnH2n+2
where,
n=number of C-atoms
Examples: methane , ethane, propane , butane etc.
Type of reactions: Characteristic reactions of alkanes are “substitution reactions”. Alkanes are relatively chemically inert to some extent that’s why they are also called paraffins.
Alkenes
Alkenes are open chain unsaturated hydrocarbons in which a carbon-carbon bond is a double covalent bond.
General formula: CnH2n
Where, n=number of carbon atoms and n is greater than 1.
They are very reactive organic compounds due to the presence of PI-BOND. They are also known as olefins.
Type of reactions: Characteristic reactions of alkenes are “addition reactions”.
Examples: ethene, propene etc.
AlkynesAlkynes are open chain unsaturated hydrocarbons in which one bond between any two carbon atoms is a triple covalent bond.
General formula: CnH2n-2
Where, n=number of C-atoms and n is greater than 1.
Alkynes are more unsaturated hydrocarbons than alkenes. They are also very reactive compounds.
Type of reactions: Characteristic reactions of alkynes are “addition reactions”.
Examples: ethyne, propyne etc.

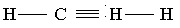

Top comments (0)