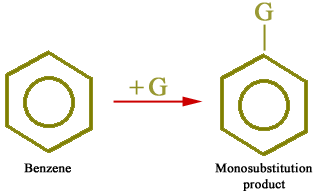
All hydrogen atoms of the benzene ring are equivalent. Therefore, when a group enters into the benzene ring, only one monosubstituted product (C6H5-G) is possible as described below:
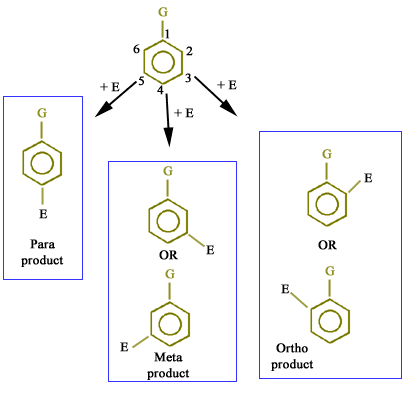
Problem starts when another group (E+) or second substituent enters into monosubstituted benzene and it can occupy any one of the remaining five positions as shown below:
But which one of the five positions the new group will occupy??? Let us discuss the exact possibility.
Effect of substituents on further substitution
Positions 2 and 6 are same (ortho product)
Positions 3 and 5 are same (meta product)
Position 4 is para position
Influence of substituent
A substituent (G) already present on the benzene ring imparts two types of influence on benzene ring for further substitution.
- Directive effect
- Activity effect
Directive or orientation effect
The first substituent (G) may direct the next incoming group (E+) to ortho, meta or para position, depending on the nature of the first substituent. This is called directive or orientation effect.
Activity effect
The substituent already present may activate or deactivate the benzene ring towards further substitution. These effects are called the activity effects.
Directive effects of substituents
In monosubstituted benzene, C6H5-G, there are five hydrogen atoms.
Ortho = 2 positions
Meta = 2 positions
Para = 1 position
But, this distribution is never obtained. The products formed, in fact, is determined by the nature of the first substituent already present on the ring.


Top comments (0)