Nucleophilic substitution reactions
Definition
A reaction in which a nucleophile displaces another nucleophile
and takes its position is called a nucleophilic substitution reaction.
Nucleophilic substitution reaction are represented by the symbol “SN“.
Explanation
In alkyl halides, the C-X bond is polar because C-atom is attached to a highly electro-negative halogen atom. Therefore, in alkyl halides carbon atom is electrophilic and halogen has a nucleophilic character.
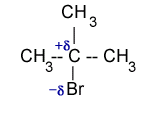
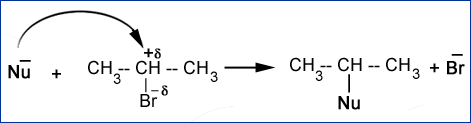
For example in iso propyl bromide, C-atom has partial positive charge and Br-atom has partial negative charge. The C-atom has capability to accept a pair of electrons to form a new bond. In doing so the C-Br bond breakes and Br– leaves the molecules. The net result is that one nucleophile displaces another nucleophile. Such displacement reactions are called nucleophilic substitution reactions.
Following examples illustrate the nature of “SN” reactions:
OH– + CH3+-I– à OH – CH3 + I–
In this example hydoxyl ion is an attacking nucleophile while iodide-ion is called leaving group.
CH3-CH2-I + OH– àCH3-CH2-OH +I–
CH3-I + –OCH3 àCH3-O-CH3 +I–
CH3-Br + CH3COO– àCH3COOCH3 + Br–
General expression
A nucleophilic substitution reaction (SN).
Nu– + Rd+-Xd- à Nu-R + X–
In general it can be written as:
Alkyl halide + nucleophile à new compound + halide ion
Mechanism of SN reactions
There are two classes of SN-reactions:
- SN1-reactions
- SN2-reactions
SN1-reactions
Nucleophilic substitution reactions which take place in two steps are referred to as “SN1-reactions.
Mechanism
SN1 mechanism occurs in two steps. In the first step, the substrate ionizes slowly and reversibly to produce carbonium ion.
R-X àR+ + X–
In the second step, nucleophile reacts with carbonium ion to produce a new compound.
R+ + Nu– à R-Nu
Rate of SN1-Reactions
The rate of an SN1 reaction depends upon the concentration of substrate (R-X) only and not on the nucleophile.i.e.
Rate = K[R-X]
This means that it is a unimolecular reaction. Therefore, SN1 is a unimolecular nucleophilic substitution reaction.


Top comments (0)