VERIFY BOYLE’S LAW WITH THE HELP OF K.M.T
According to Boyle’s law at constant temperature, pressure & volume of a gas are related as:
PV = constant
According to Kinetic theory, pressure of a gas is
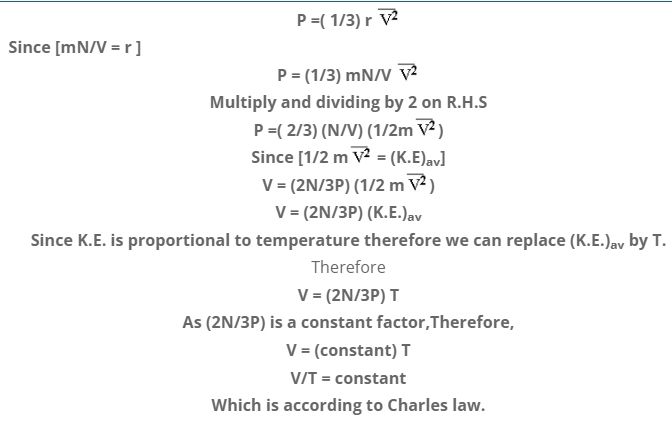
VERIFY CHARLES LAW WITH THE HELP OF K.M.T
According to Charles law at constant pressure, absolute temperature & volume of a gas are related as:
V/T = constant
According to Kinetic theory, pressure of a gas is



Top comments (0)