Chemical Kinematics is a branch of physical chemistry that deals with determination of rate of reaction. A reaction proceeds with disappearance of a reactants and formation of products. So, the rate of a chemical reaction can be measured in terms of rate of disappearance of reactants and formation of products.
Rate of reaction:
= mols litre-1 time -1
For reaction
A + B → C
Rate of reaction can be measured in terms of rate of disappearance of A and B and rate of formation of C.
Rate of disappearance of B:
Rate of disappearance of C:
Note: -ve sign is used in change in concentration of reactant to make rate of reaction positive.

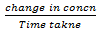
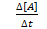
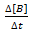
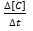

Top comments (0)