1. Concentration:
Haematite ore being oxide ore is concentrated by gravity separation process using hydraulic separator. The oxide is being heavier settles to the bottom while lighter impurities come to the surface and is removed.
2. Calcination:
The concentrated ore is heated in limited supply of air on the shallow hearth of Reverberatory furnace during calcination, flowing changes occur.
- Moisture and volatile impurities are driven out.
Non-metallic impurities like s, p, etc are removed as their oxides.
S + O2 → SO2
P4 + 5O2 → 2P2O5If any carbonate is present then it dissociates to give oxide.
FeCo3 → FeO + Co2
3. Smelting:
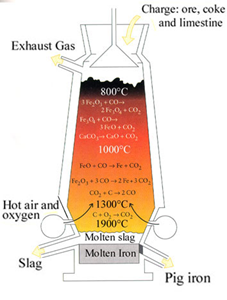
The calcined ore is mixed with lime stone (CaCO3) (Flux) and ore in the ratio of 8:1:4 and charged into blast furnace. The blast furnace is cylindrical steel vessel limed 6m in diameter. On passing Pre heated compressed air inside the furnace, four distinct zones are formed.
Zone of reduction combustion:
This zone lie at the bottom of the blast furnace where carbon burns in presence of pre heated air forming CO2 and heat.
C + O2 → Co2 + heat
Here, the temp is maintained above 15000c. This produces the energy required for the smelling of iron.
Zone of fusion:
This zone lies above zone of combustion. Here upcoming CO2 is reduced by red-hot coke to carbon monoxide. The reaction is endothermic and temp falls to 12000 -13000 c
Co2 + C → 2CO – heat
Red-hot
The spongy iron formed in zone of reduction melts here and any oxide escaped reduction is reduced here.
2Fe2O3 + 3C → 4Fe + 3CO2
Zone of slag formation:
This zone lies above zone of fusion and it has temp of about 10000c. In this zone calcium carbonate dissociate forming can (calcium oxide) which combines with silica (SlO2) present as impurity in Haematite forming calcium silicate (CaSiO3) as slag.
CaCo3 → CaO + CO2
CaO + SiO2 → CaSiO3
Calicum flicate (slag)
The slag is lighter than molten iron and to floats on the surface of the iron. The formation of prevents the oxidation of iron.
Zone of reduction:
This is the most important zone and has temperature of 600-7000c. In this zone Fe2O3 is reduced to iron by co in three steps.
3Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + CO → 3FeO + CO2
FeO + CO → Fe + CO2
(Solid state)
From the bottom of blast furnace, molten slag and molten iron are tapped out. This iron is impure and is called cast-iron or pig-iron.


Oldest comments (0)