1. Concentration
Cinnabar being sulphide is concentrate by froth floatation method. The pulverized are is kept in water containing pine oil & the mixture is agitated by passing compressed air. Ore forms froth with oil and come to the surface and are skimmed off while impurities are left in water.
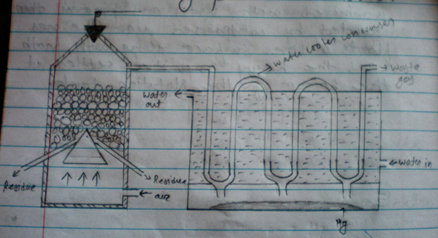
2. Roasting and distillation
The concentrated are is mixed with 2% coke and fed into shaft furnace through a cup and wine. Arrangement the furnace is heated by pruning fuel and air is below in cinnabar is first oxidized to mercuric oxide which then decompose into mercury the vapor of mercury are condensed in Y-shaped. Earthen condensers cooled by water.
2HgS + O2 → 2HgO + 2SO2
2HgO → 2Hg + O2
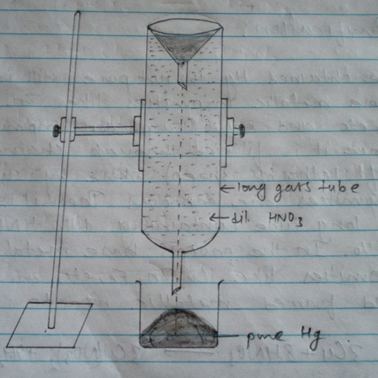
3. Purification:
Mercury obtained by above process is not pure and contains impurities like copper (Cu), Zn, Bi, Ag, etc. as impurities. The impure mercury is filtered through daemons leather to remove suspended soiled. After that mercury is dropped through a long tube filled with 5% HNO3 solution. The base metals dissolve in HNO3 as Nitrate.
4Zn + 10 HNO3 → 4Zn(NO3)2 + NH4NO3 + 3H2O
4Cu+ 10 HNO3 → 4Cu(NO3)2 + 2NO + 4H2O
Any mercurous nitrate if from reacts with base metal giving back mercury.
Hg2(NO3)2 + Cu → Cu (NO3)2 + 2Hg
Mercury still contains metal like gold (Au), silver (Ag), Pt, etc. as impurities. These are removed by vacuum distillation where mercury distills leaving other metals.
Properties:
- Mercury is a shining metal having density 13.6 gmcm3
- It has mpt on -370c and bpt 3570c
- It vapourises forming monoatomic mercury molecule and mercury vapour are highly poisonous.
Chemical properties
Action of air
Air does not react with Hg, at normal (ordinary) temperature but on heating, mercury to its boiling point combines with oxygen forming mercuric oxide.
2Hg + O2 → 2HgO
Action of ozone
In an atmosphere of ozone, mercury loses its meniscus due to formation of mercurous oxide. When this mercury is allowed to slide down on glass surface, it leaves mercurous oxide in its trail. This phenomenon is called Tailing of Mercury
2Hg + O3 → Hg2O + O2
Mercurous oxide
Action of acids
dil. HCl and Dil H2SO4 does not reach while dil. HNO3 gives mercurous nitrate.
6Hg + Dil8HNO3 → 3Hg2(NO3)2 + 2NO + 4H2O Mercurous nitrate
Hot and conc. H2SO4 gives SO2 gas along with mercuric sulphate.
Hg + 2H2SO4 → HgSO4 + SO2 + 2H2O
conc.
Mercurous sulphate
Hot and conc. HNO3 gives mercuric nitrate and Nitrogendioxide.
Hg + 4HNO3 → Hg(NO3)2 + 2NO2 + 2H2O
conc.
Conc. HCl alone does not react with mercury but aquaregia dissolves mercury as mercuric (Corrosive sublimate)
HNO3 + 3HCl → NOCl + 2H2O + 2Cl
Hg + 2Cl → HgCl2
Hg + HNO3 + 3HCl → NOCl + HgCl2 + 2H2O
Action of halogen:
Mercury combines directly with halogen to form mercury halides. The compound depends upon availability of halogen.
Hg + Cl2 → HgCl2
Excess Mercuric chloride
2Hg + Cl2 → Hg2Cl2
Limited Mercurous chloride
Action with sulphur
Mercury combines readily with sulphur at ordinary temperature forming mercuric sulphide.
Hg + S → HgS
Uses of mercury
- It is used in making thermometer, barometer etc.
- It is used in making mercury vapour lamps.
- It is used in extraction of gold by amalgumation process.
- It is used in manufacture of Caustic Soda (NaOH) by castner kellner process and kellner solvay process
- It is used in making amalgam.


Top comments (0)