The chief are of silver is argentite and silver is extracted by hydrometallurgy process. The silver are is dissolved in cyanide solution to form soluble argento cyanide complex from which metal is obtained by reduction with more electropositive. The different steps involved in extraction of silver are:
1. Ore – Concentration:
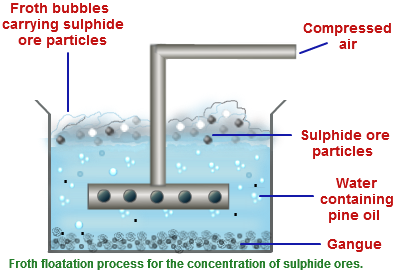
Argentite being sulphide ore and is concentrated by froth flotation process. The pulverized ore is kept in large tank containing water and pine oil. The mixture is disturbed by passing compressed air where ore forms froth with pine oil & comes to the surface while impurities are left in water.
2. Treatment with sodium cyanide:
The concentrated are is treated with 0.4% to 0.7% aqueous solution of sodium cyanide and the current of air pass through it. The argentite ore dissolves in sodium cyanide forming sodium argento cyanide.
Ag2S + GNaCN → 2Na [Ag(CN)2] + NO2S
Sod argento cyanide
The reaction is reversible so is passed to oxidize Na2S to Na2SO4 such that the equilibrium shifts towards product.
Na2S + O2 → Na2SO4
The solution is filtered and the filtrate containing sodium argento cyanide is used to recover silver metal.
3. Precipitation of silver:
The solution obtained is treated with Zn scrap where Xn displaces silver from its complex.
Zn + 2Na [Ag (CN)2] → NO2[Zn (CN)4] | 2Ag
Sod Zincyanide
The ppt is collected, washed and fused to get compact mass of silver.
Refining:
The impure silver is purified by electrolytic method. A block of impure metal is anode while a thin strip of pure silver is cathode. A mixture of AgNO3 solution is electrolyte. On passing current impure silver dissolves and equivalent amount of pure silver is deposited at cathode.
AgNO3(aq) → Ag+ + NO–3
At cathode: Ag+ + e → Ag
At anode: Ag → Ag+ +e
Purity of Silver
The purity of silver is expressed in terms of fitness. Fitness of silver is mass of pure silver in grams present in every 1000 gram of silver sample. For eg. The fitness of silver in 900 means 900 gm of pure silver is present in 1000 gram of sample of silver.
In case of gold, purity is expressed in both fitness as well as carat. A 100% pure gold simple has purity of 24 carat. For eg. 18 carat gold is (18/24 × 100%) = 75% pure and has fitness 750.
Properties
- It is a white lustrous metal having mpt 9600c and bpt 22120c.
- It is sp gravity 10.5 and is highly malleable and ductile.
- Silver is not affected by water, alkali and non-oxidising acids.
- Action of HNO3
Silver dissolves in both dilute and conc. HNO3 having silver nitrate.
3Ag + 4HNO3 → 3AgNO3 + NO + 2H2O
Ag + ConC2HNO3 → AgNO3 + NO2 + H2OSilver is readily attacked by sulphur and H2S to form a black stain of silver sulphide. (Ag2S)
Uses
- Silver is used in making ornaments, coins, decorative articles etc.
- Silver is used in electroplating of silver, making silver mirror etc.


Oldest comments (0)