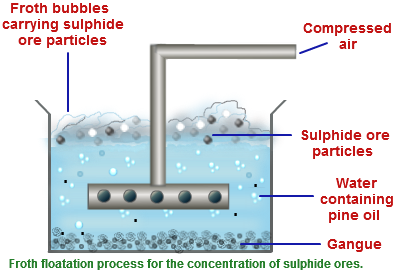
Concentration:
Zinc blende is concentrated by floath floatation process. The pulverized ore is kept in large tank containing water and pine oil. The mixture is agitated by passing compressed air. Ore forms froth and comes to the surface while impurities are left in water.
Roasting:
The concentrated are is heated in excess supply of air above 9000c on the hearth of reberveratory furnace. During roasting. Zinc sulphide is converted to Zinc Oxide.
2ZnS + 3O2 → 2ZnO + 2SO2
Small amount of ZnS may be oxidized to ZnSO4 but above 9000c , ZnSO4 decompose forming Zinc Oxide (ZnO)
ZnS + 2O2 → ZnSO4
ZnSO4 above 900 → 2ZnO + 2SO2 + O2
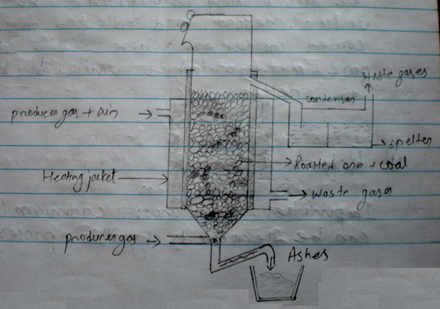
Reduction (Smelting):
ZnO obtained during roasting is mixed with coke and heated strongly where ZnO is reduced to Zn by carbon.
ZnO + C → Zn + CO
The reduction is done in vertical refort. In this process roasted are mixed with coke in the ration of 2:1 and small briquets are made. These briquets are fed into vertical report furnance, from the charging door. The report is heated externally by burning produce gas (w+N2) to about 14000c. The vapour of zn is camed to condenser where it condenses to give molten zinc called spelter zinc.
Purification:
Zinc spelter contains pb, fb, cd, as, etc. as impurities. Impure zinc can be purified by following methods.
1. By fractional distillation:
The bpt of Pb, Fb are higher than that of zinc while that of cadmium, arsenic are lower than that of zinc. When distillation is carried out around 1000°c, zinc, Cd, As, etc. distill off leaving Pb and Fe the distillate is then heated to 800°c where cd and as distill off leaving pure zinc. This sample of Zn is about 99% pure.
2. By electrolysis:
Zinc of higher purity can be obtained by electrolysis. Pure zinc rod is used as cathode while a block of impure zinc is used as anode. A mixture of ZnSO4 and dill H2SO4 is used as electrolyte. On passing current impure zinc dissolves and equivalent amount of pure zinc is deposited at cathode.
Physical properties
- It is a blueish white lusticous metal.
- Zinc is brittle at ordinary temperature but it becomes malleable from 100-150. c then again it becomes brittle.
- It melts at 420. c and boils at 900. c and has sp. Gravity 7.13.
Chemical properties
1. Action of air:-
Dry air has effect on zinc but in moist air, zinc forms a protective layer of basic zinc carbonate.
Zn + O2 + H2O + CO2 → ZnCO3.Zn (OH)2
When heated in air, zinc burns greenish blue flame forming clouds of light, white power of zinc oxide which is commonly known as ‘philosopher’s wool. Beside this name, other common names are ‘Zinc white and china white.
2Zn + O2 → 2ZnO
2. Action of water:-
Pure zinc does not react with water but impure zinc displaces hydrogen gas. Zn-inc reacts with hno3 in four different concentrations giving different reduced product.
Zn + H2O → ZnO + H2
3. Action with acids:
Dilute H2SO4 and dil. HCl gives H2 gas with zinc
Zn + dil. H2SO4 → ZnCl2 + H2
Zn + dil. HCl → ZnSO4 + H2
With hot and conc. H2SO4 zinc gives SO2 gas
Zn + 2H2SO4 → ZnSO4 + SO2 + 2H2O
Zinc reacts with HNO3 in four different conditions giving different product:
With very dil. HNO3
With very dil. HNO3, Zn gives ammonium nitrate
4Zn + 10HNO3 → 4Zn(NO3)2 + NH4NO2 +3H2O
With dil. HNO3
Zinc reduces dil. HNO3 to nitrous oxide (N2O)
4Zn + 10HNO3 → 4Zn(NO3)2 + N2O +5H2O
With modrately conc. HNO3
Zinc reduces moderately conc. HNO3 to NO (nitric oxide)
3Zn + 8HNO3 → 3Zn(NO3)2 + 2NO2 +4H2O
With conc. HNO3
Zinc reduces conc. HNO3 to NO2 (nitrogen dioxide)
Zn + 4HNO3 → Zn(NO3)2 +2 NO2 +2H2O
4. Reaction with Alkalies:-
Zinc readily dissolve in hot and conc. solution of caustic bases like NaOH , KOH, etc giving hydrogen gas.
Zn + NaOH → Na2ZnO2 + H2
Zn + KOH → K2ZnO2 + H2
5. Displacement Reaction:-
Zinc can displace less electropositive metals from their salt solution
Zn + CuSO4 → ZnSO4 + Cu
Zn + 2Na[Au(CN)2] → NA2[Zn(CN)4] + 2Au
Uses of zinc:
- It is used for galvanization of iron.
- It is used in making alloys like Brass. German metal, German silver, etc
Galvanization
The process of applying a coat of zinc on base metal like iron is galvanization Iron is galvanized to protect iron from resting the galvanization is done in following steps.
Cleaning and picking:
Before applying zinc coat on iron, the surface of iron should be clean. The cleaning is done first by sand blast and then washed by dipping in diluted acid. The process of cleaning iron by using dil. acid is called picking.
Applying of zinc coat:
The coat of zinc can be applied on iron surface by 3 methods.
By electroplating:-
Zinc can be electroplated on iron by keeping iron article as cathode, zinc as anode and znso4 solution as electrolyte. On passing current a layer of zinc is coated on iron article.
By metallizing:-
Zinc can be directly applied by dipping iron article in molten zinc this process is generally used for galvanization of iron sheets. Iron sheets are dipped in molten zinc bath & then passed through huge rollers that distributes zinc coat equally.
By sherardizing:-
The process is used for galvanization of small iron articles like nail, screws etc. In this process iron article are mixed with zinc dust and then heated in enclosed vessel for few hours when coat of zinc is applied on iron.


Top comments (0)