The different factors that affect the rate of reaction are:
Concentration: In general, the rate of reaction is directly proportional to concentration of reactants. When concentration is increased, the no. of molecules increases which caused increase in no. of collisions that can give rise to product. However, the rate of reaction is independent of concentration of reactant for 0th order reaction.
Size of particle: The rate of reaction increases with decrease in size of particle. Smaller particle have higher exposed surface area and more molecule can involve in collision to give products.
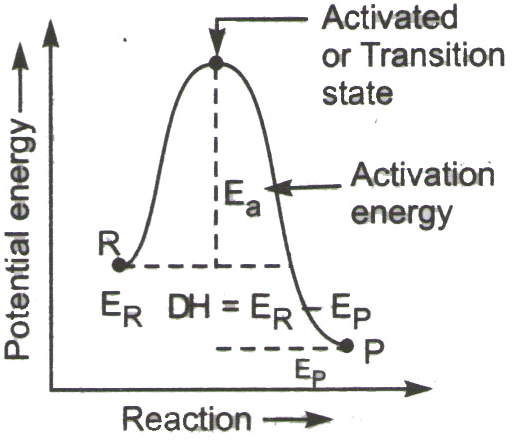
Temperature: When temperature is increased, the average energy of reacting molecule increases. This causes increase in fraction of reacting molecule possessing activation energy. So the increase in temperature causes increase in rate of reaction. The distribution curve of no. of reactant molecule with energy at different temperature is as follows:
In the distribution curve it is observed that the no. of reactant molecular that possess activation energy gets almost double during increase in temperature by 100C in general the rate of reaction increases by 2-3 times for every 100C rise in temperature.
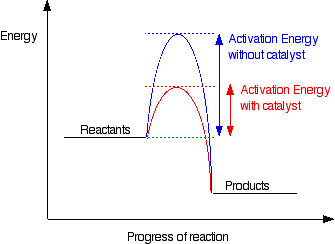
Catalyst: Catalyst is a chemical substance that alters the rate of reaction without itself being involved in the reaction. A positive catalyst increases the rate of reaction by providing an alternate path of reaction which has smaller activation energy. When activation energy is decreased more reactant molecule can cross the energy barrier to form products.


Latest comments (0)