The different gas laws were initially started by scientist on the basis of experimental result. There was no theoretical background to explain these laws. In order to describe the different gas laws a theoretical model was developed which was based on the molecular concept of matter and kinetic concept of gases. This theory is known as kinetic theory of gases.
It was originally presented by Bernoulli and further extended by Clausius, Max-Well and Boltzman. The main postulates of this theory are as follows.
- Gas are composed of very minute particles known as molecules
- Gas molecules are separated from each other by large intermolecular distance with huge empty space between them. So, the volume occupied by gas molecules is negligible in comparison to the volume of the gas.
- There is negligible force of interaction between the gas molecules.
- The gas molecules are in a state of constant motion. During their motion, they collide among themselves as well as with the walls of the container.
- The molecular collisions are perfectly elastic i.e. there is no loss of kinetic energy during their collision.
- The pressure of the gas is due to the bombardment of gas molecules on the walls of the container.
- The average kinetic energy of gas molecules is directly proportional to the temperature in Kelvin scale.
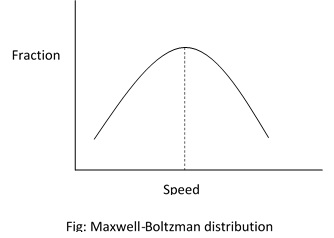
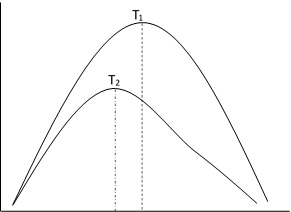
- The molecules of gases are moving with different speeds. However, there exists certain pattern of distribution of speed among the molecules. This is known as Maxwell-Boltzman distribution.
It is found that the fraction of molecules bearing large velocity as well as the fraction of molecules bearing small velocity are both same. Most of the molecules have velocities which are intermediate between the maximum and minimum. The velocity with which the largest fraction of molecules is moving is known as most probable speed. Its value increases with increasing temperature.


Top comments (0)