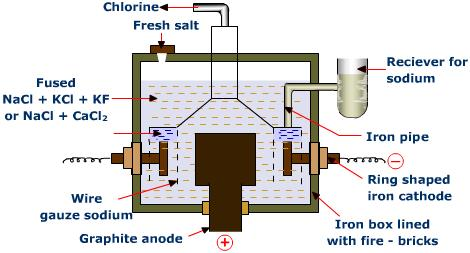
Principle:
Down’s cell is a special type of cell that is made for extraction of sodium. During the extraction of sodium metal, following difficulties are seen:
- Sodium ores like NaCl, Na2CO3 are very stable.
- The chemical reduction of those sodium salts is not possible since sodium itself is a good reducing agent.
- The electrolytic reduction of its aqueous solution is also very difficult because H+is reduced faster than Na+ and hence H2 is produced before than Na.
- Reducing of its molten salt by electrolysis is also very difficult because the melting point of NaCl is around 800°C but that of sodium metal is 840°C which can be corrosive for the cell causing the fog of sodium and there is the chance of short circuit also.
Hence, to overcome such difficulties, the Down’s cell was brought in the use. NaCl was mixed with CaCl2 in the ratio of 2:3 by mass which reduces the working temperature to 600°C. This cell consists of graphite anode & iron cathode. The cell consists of one inlet through which the electrolyte is poured into the cell. Then the electricity is passed into the electrodes which initiate the electrolysis. The chlorine gas collected at anode escapes through the hood as byproduct and the sodium metal is extracted in the cathode. Sodium, having low density than its salt, floats and can be easily collected over kerosene.
At anode:
Cl–– e– ——–> Cl
2Cl ———>Cl2
At cathode:
Na++ e– ———> Na


Top comments (0)