By Bessemerisation process:
Principle:
In bessemerisation process, the impurities present in cast iron are removed by air oxidation and calculated amount of carbon is added in the form of spigelesien (Fe + Mn +C) is added to obtain Steel. The lining of Bessemer converter depends upon presence or absence of phosphorous. If phosphorous is present, then basic lining of CaO or MgO is used and if phosphorous is absent, then acidic lining of SiO2 is used. The reactions occurring during manufacture of steel are.
2C +O2 → 2CO
S + O2 → SO2
Si + O2 → SiO2
2Mn + O2 → 2Mno
MnO + SiO2 → MnSiO3
Manganous silicate (slag)
If phosphorous is present,
4P + 5O2 → 2P2O5
P2O5 + 3CaO → Ca3(PO4)2
Calcium phosphate (thomas slag)
Process:
Molten cast iron is kept in Bessemer converter lined internally by basic lining (if phosphorous is present) or acidic lining (if Phosphour is absent) and blast of air is passed into the mixture. At first impurities other than carbon are oxidized. Then carbon is oxidized to carbon monoxide which burns with light blue fame at the mouth of bessemer converter. When the flame dies out, calculated amount of spigelesin is added and mixed by passing air for some time. Then steel is manufactured. The process is completed in 15-20 minutes and the batch size is 500-600Kgs. The quality of steel obtained by Bessernerization process is not uniform. During the process some amount of Iron is lost as slag.
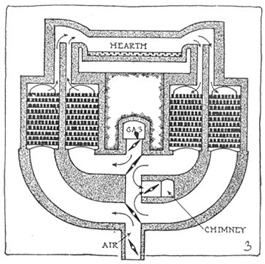
By Siemen Martin’s process or open hearth process:
Principle:
In open hearth process, the impurities present in cast iron are removed by oxidation by haematite. The percentage of carbon is decreased by adding scrap iron. The heat required for the process is obtained by burning per-heated producer gas (Co+N2) by regeneration of heat economy Depending upon the impurities the lining of hearth is acidic (If phosphorous is absent) or basic lining (if phosphorous is present). The percentage of carbon is maintained by adding required amount of splgelesien. The reactions during manufacture of steel are.
3C + 2Fe2O3 → 4Fe + 3CO2
3S + 2Fe2O3 → 4Fe + 3SO2
3Si + 2Fe2O3 → 4Fe + 3SiO2
SiO2 + Cao → CaSiO3
If phosphorous is present,
6P + 5Fe2O3 → 10Fe + 3P2O5
P2O5+3CaO → Ca3(PO4)2
Process:
Mixture of cast iron scrap iron , haematite and small amount of lime is kept on hearth of open hearth furnace & mixture is healed by burning producer gas by regenerative system. The outgoing hearth gas pre. Heats the incoming producer gas which an combustion can generate higher temperature. The slag formed is removed and a small amount of steel is withdrawn from the hearth and is analyzed. The percentage of carbon in steel can be increased by adding spigelicien and can be decreased by adding scrap iron. The process is slow and takes about 8-10 hours and quality steel is better as sample can be analyzed.
The advantages of open hearth process over bessemerization process are as follows:
- The quality of steel is uniform.
- Substance like Haematite, scrap iron is converted to valuable steel.
- The loss of iron is very small.
- The batch size in open hearth process is very large compared to bessernerisation process.


Top comments (0)