Let’s consider a reaction
N2 + 3H2 → 2NH3
The rate of reaction can be expressed as,
Here, during disappearance of 1 mole of N, 3 mols of H2 are disappeared. So, the rate of disappearance of H2 is 3 times the rate of disappearance of N2.
The disappearance of 1 mole of N2 gives 2 moles of NH3. So the rate of formation of NH3 is 2 times the rate of disappearance.
The equivalent rate of reaction is,
Therefore, the equivalent rate of a reaction is obtained by dividing the rate of each species by its stoichiometric coefficient,
aA + bB → cC + dD
The equivalent rate of reaction is,
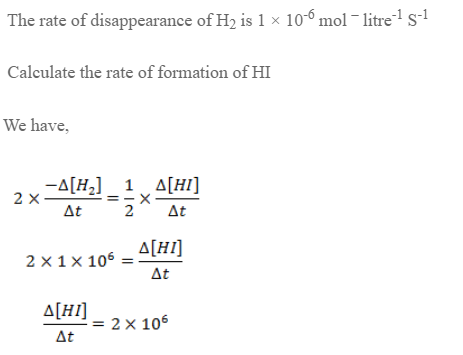
For the reaction,
H2 + I2 → 2HI
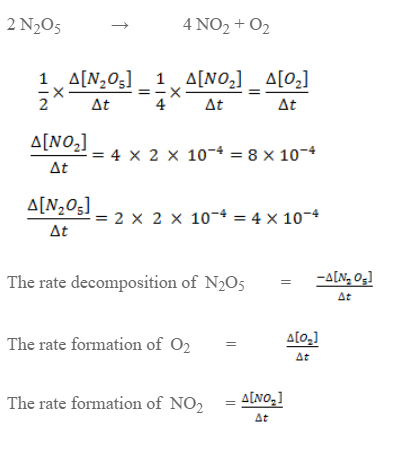
Express the rate of reaction in terms of all species for decomposition of N2O5. If the rate of formation of O2 is 2× 10-4 mol lit-1 S-1 calculate the rate of disappearance of N2O5 and rate of formation of N2.

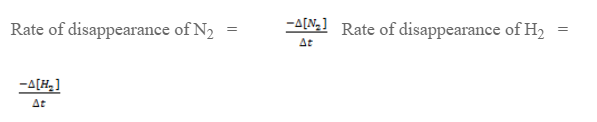

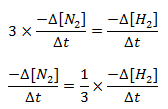
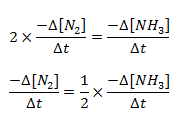
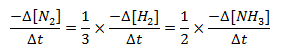
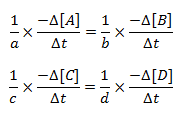

Top comments (0)