In thermodynamics sense, a process that occurs by itself without the help of external agent is a spontaneous process. All naturally occurring process is spontaneous process.
Eg:
- Rolling down of a stone downhill
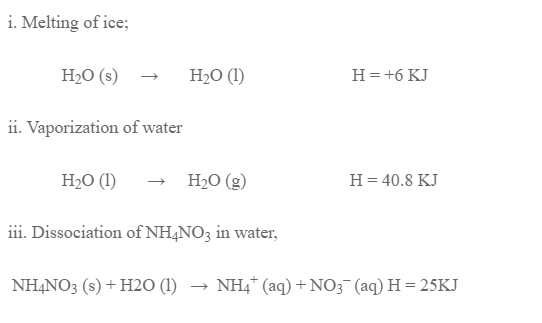
- Melting of ice
- Flowing of water from higher to lower level
- Vaporization of water
- Burning of fuel
- Mixing of gases
- Natural radioactivity, etc.
Characteristics of spontaneous process:
- All spontaneous process are unidirectional in nature and processed till the process is completed or a state of equilibrium is reached.
- Some spontaneous process can be reversed by applying external agency.
- Some spontaneous process requires initiations such energy is called activation energy.
What is the driving force for spontaneous of a process?
In the early development of thermodynamics energy change was considered as criteria for spontaneity a process occurs with decrease in energy is spontaneous. eg. flowing of water from higher to lower level, rolling down of stone downhill etc. In case of chemical reaction, the reaction that occurs with decrease in enthalpy i.e. exothermic is spontaneous like burning of Methane, neutralization reaction, etc. On the basis of enthalpy change,
H = -ve = exothermic = spontaneous
H = +ve = endothermic = non-spontaneous
H = O = equilibrium
However, many endothermic reaction were found to be spontaneous. Eg. Melting of ice
These examples show that enthalpy change alone is not an enough criteria for spontaneity. These lead to necessity of another thermodynamic function and thermodynamic law.
In all above examples, the molecular disorderness is increased so the new thermodynamic function should measure disorderness. This function is called entropy.


Latest comments (0)